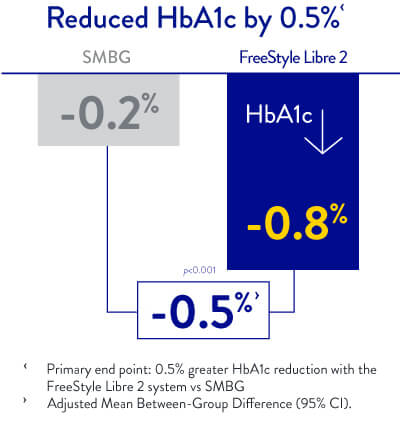
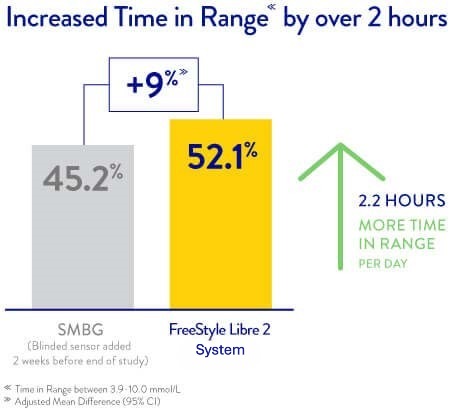
* Sale of the original FreeStyle Libre system has been discontinued in the UK market. The FreeStyle Libre 2 and 3 systems are for sale, providing the same benefits as the original FreeStyle Libre system, with the added functionalities of optional Real-Time Alarms.
1. Bolinder J, Antuna R, Geelhoed-Duijvestijn P, Kr ger J, Weitgasser R. Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial. Lancet. 2016;388(10057):2254-2263.
2. Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline JP, Rayman G. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther. 2017;8(1): 55-73.
3. Leelarathna, L. N Engl J Med. (2022): DOI: 10.1056/NEJMoa2205650.
4. Yaron, M. Diabetes Care (2019): https://doi.org/10.2337/dc18-0166
5. Fokkert M, van Dijk P, Edens M, et al. Improved well-being and decreased disease burden after 1-year use of flash glucose monitoring (FLARE-NL4). BMJ Open Diab Res Care. 2019. https://doi:10.1136/bmjdrc-2019-000809.
ADC-57236 v4.0
.svg)