References & Disclaimers
Images are for illustrative purposes only. Not real patient or data.
1. Data on file, Abbott Diabetes Care, Inc.
2. Among patient-applied sensors.
3. Fokkert, M. et al. Improved Well-being and Decreased Disease Burden After 1-Year Use of Flash Glucose Monitoring (FLARE NL-4)." BMJ Open Diabetes Research and Care 7, no 1 (2019): e000809. https://doi.org/10.1136/bmjdrc-2019-000809.
4. Based on product features, including up to 15-day wear period, automatic readings every minute, accuracy data, and single-app setup with AID systems.
5. LibreView is ISO27001/27018/27701 certified and HITRUST CSF Certified.
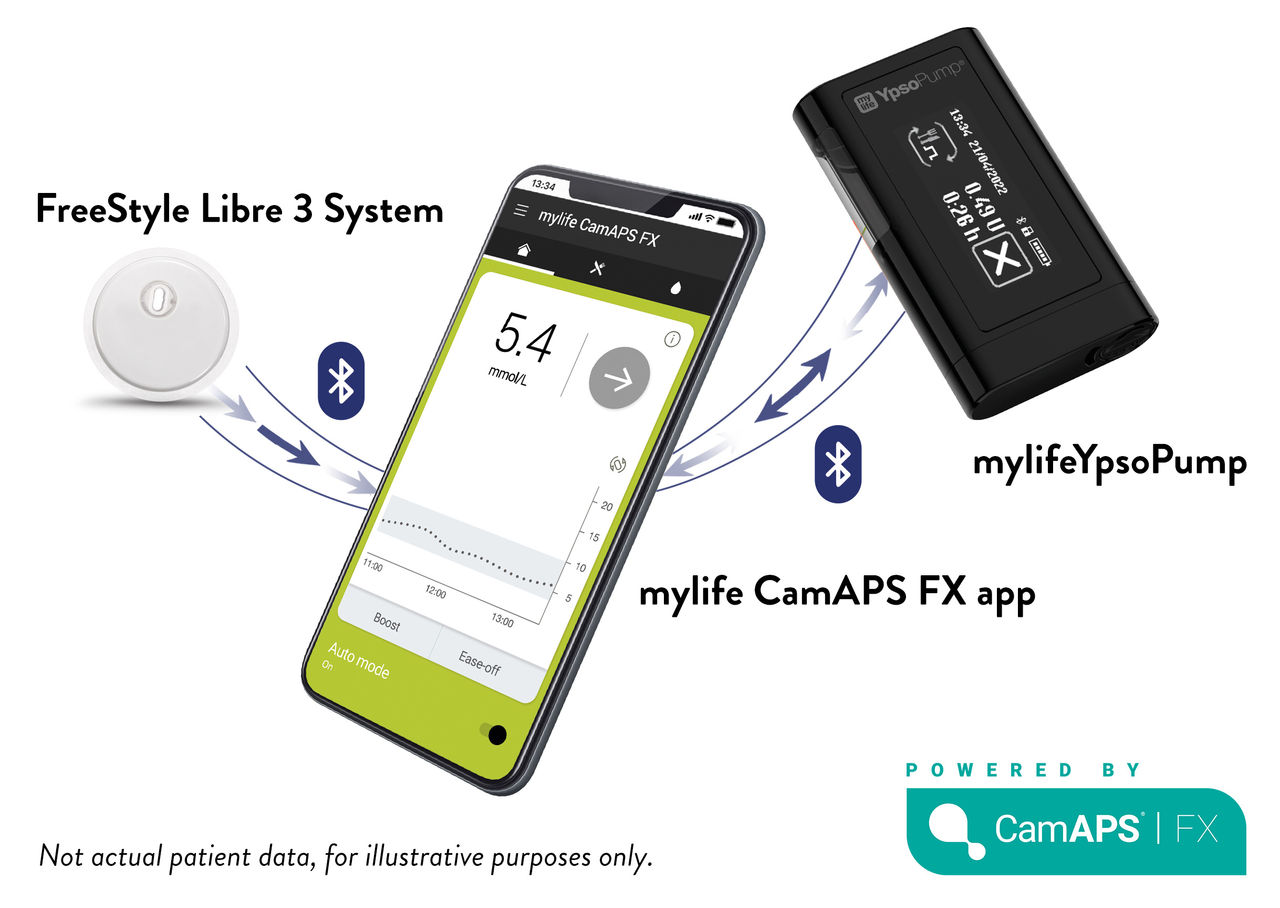
◊ The FreeStyle Libre 3 Plus sensor is authorised for use with the mylife Loop automated insulin delivery system, including the mylife CamAPS® FX app and mylife YpsoPump insulin pump. For use of the FreeStyle Libre 3 sensors with the mylife Loop, refer to the labelling provided with the mylife CamAPS® FX app.
^ For children aged 2-12, a caregiver at least 18 years old is responsible for supervising, managing, and assisting them in using the FreeStyle Libre 3 system and interpreting its readings.
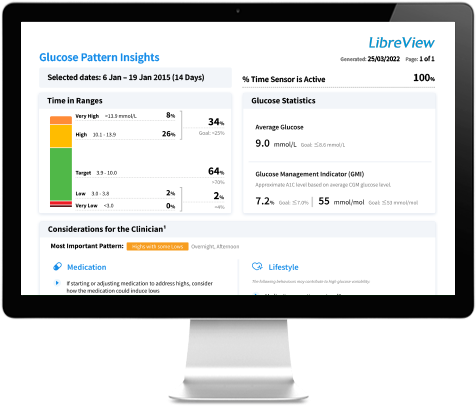
₼ The LibreView data management software is intended for use by both patients and healthcare professionals to assist people with diabetes and their healthcare professionals in the review, analysis and evaluation of historical glucose device data to support effective diabetes management. The LibreView software is not intended to provide treatment decisions or to be used as a substitute for professional healthcare advice.
ADC-119547 v1.0
.svg)