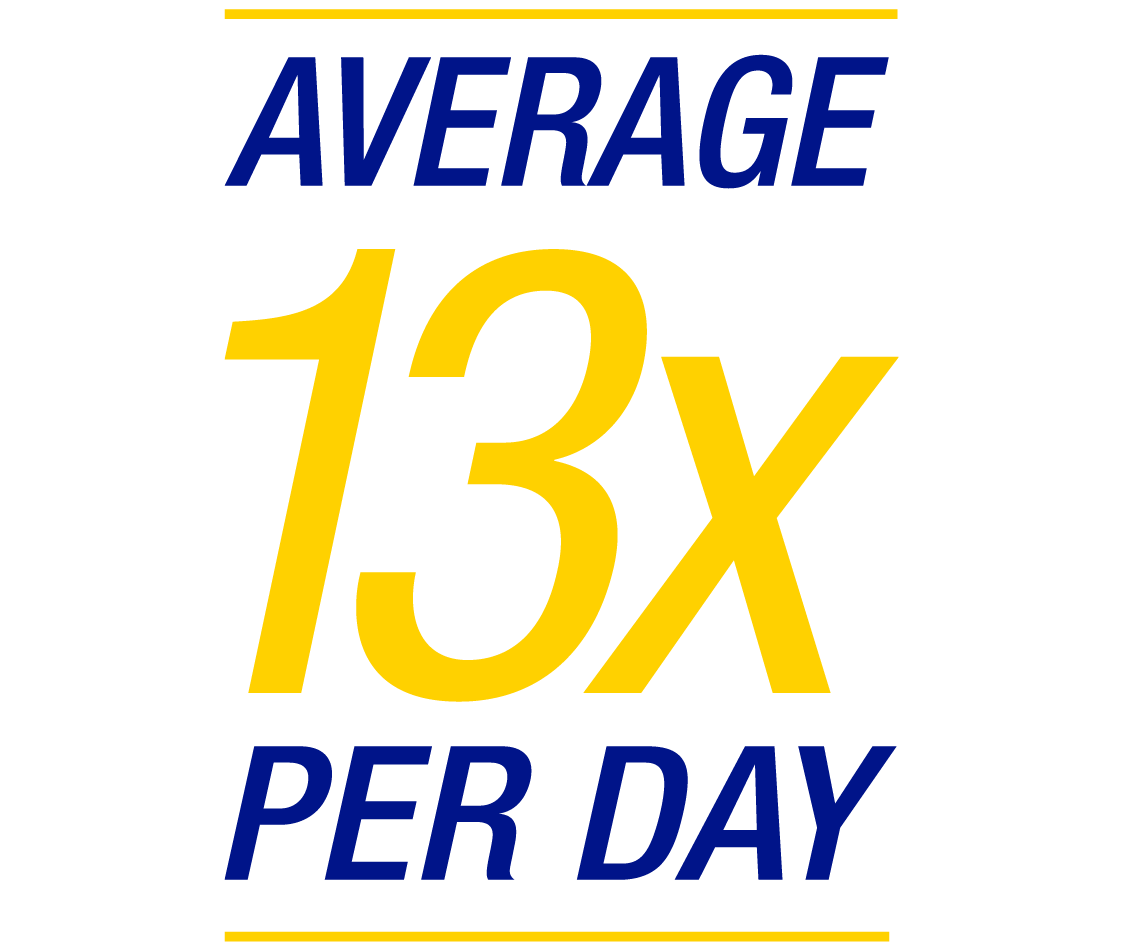An investment that pays off.
- Insulin users
- Non-insulin users
- Children with diabetes
- During pregnancy
Proven benefits for insulin users with diabetes
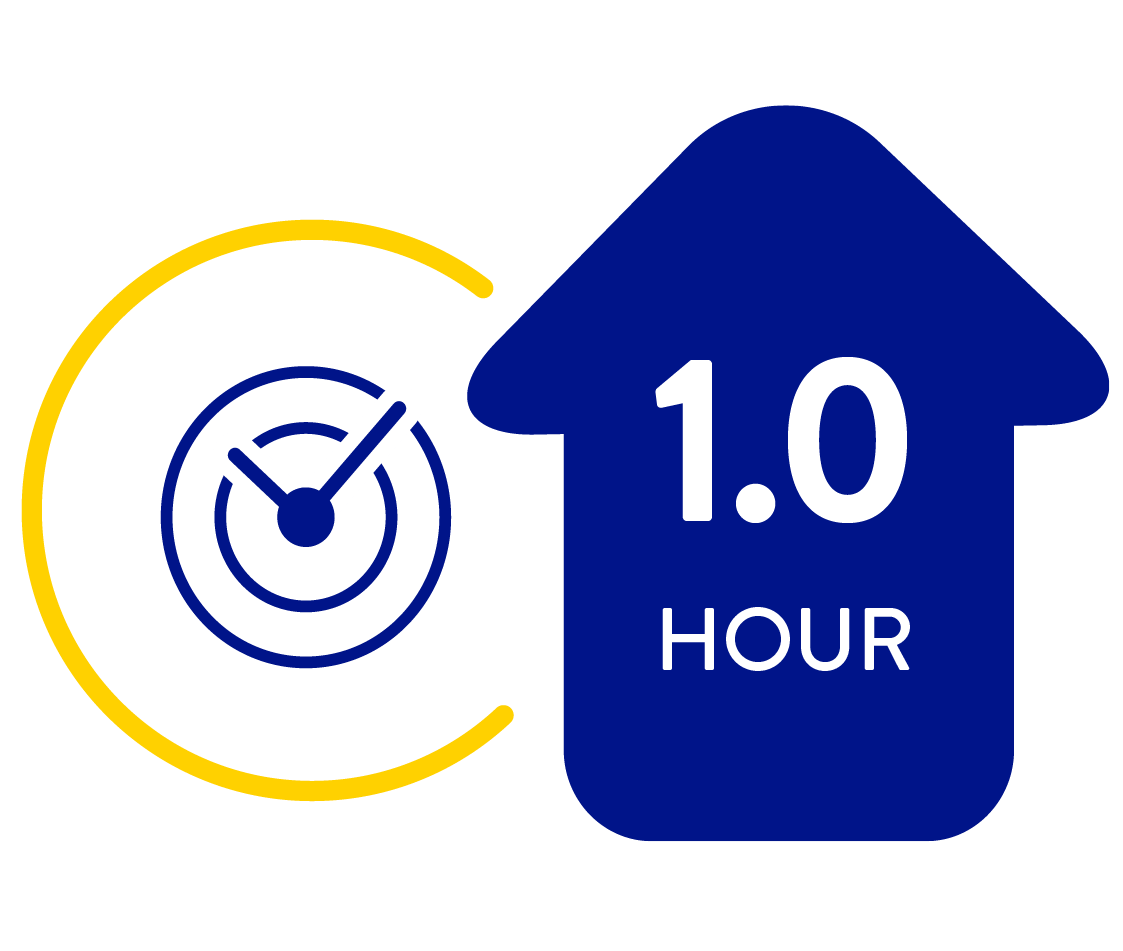
Lowering A1c reduces your risk of diabetes related complications
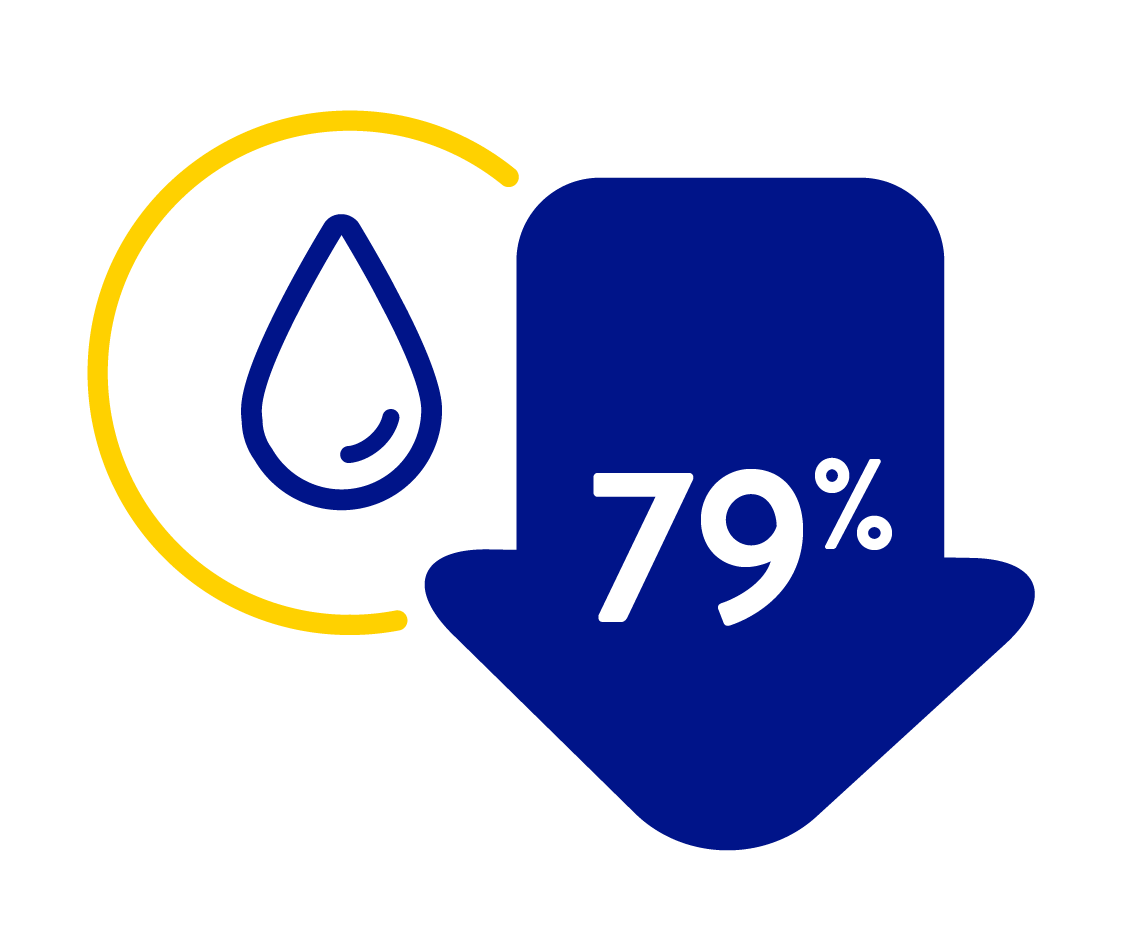
FreeStyle Libre systems have been shown to help reduce hypos in insulin-using people with both T1D and T2D28
Proven benefits in children and teenagers with diabetes210
Children and teenagers successfully improve their glucose control with FreeStyle Libre systems
FreeStyle Libre improves overall diabetes management of children and helps caregivers manage glucose levels
Parents and caregivers can rely on the FreeStyle Libre system to make treatment decisions without finger pricks10 even when glucose is low or falling
Proven results during pregnancy
FreeStyle Libre is an easy, safe, and effective way to check glucose levels for use during pregnancy for people with

Type 1 diabetes

Type 2 diabetes

Gestational diabetes mellitus

Any stage of pregnancy

Insulin therapy

Using metformin
FreeStyle Libre systems can improve overall diabetes management during pregnancy and helps you improve your glucose levels
Here’s what users are saying about FreeStyle Libre systems compared to self-monitoring blood glucose, during pregnancy205
100% agree it’s less painful
98% agree it’s less stressful
95% agree it’s more discreet
94% agree it’s easier to use
Proven benefits for insulin users with diabetes
Lowering A1c reduces your risk of diabetes related complications
FreeStyle Libre systems have been shown to help reduce hypos in insulin-using people with both T1D and T2D28
Proven benefits in children and teenagers with diabetes210
Children and teenagers successfully improve their glucose control with FreeStyle Libre systems
FreeStyle Libre improves overall diabetes management of children and helps caregivers manage glucose levels
Parents and caregivers can rely on the FreeStyle Libre system to make treatment decisions without finger pricks10 even when glucose is low or falling
Proven results during pregnancy
FreeStyle Libre is an easy, safe, and effective way to check glucose levels for use during pregnancy for people with

Type 1 diabetes

Type 2 diabetes

Gestational diabetes mellitus

Any stage of pregnancy

Insulin therapy

Using metformin
FreeStyle Libre systems can improve overall diabetes management during pregnancy and helps you improve your glucose levels
Here’s what users are saying about FreeStyle Libre systems compared to self-monitoring blood glucose, during pregnancy205
100% agree it’s less painful
98% agree it’s less stressful
95% agree it’s more discreet
94% agree it’s easier to use
You might also like: