No Fingersticks
Painless to apply*†2,7 and comfortable to wear*†2,7, without the need for fingersticks‡§
The FreeStyle Libre systems are now widely available to people on Medicare managing diabetes with insulin¶3
CGM = continuous glucose monitoring
CGM systems designed with you in mind
FreeStyle Libre systems are the #1 CGM brand in the US††
Painless to apply*†2,7 and comfortable to wear*†2,7, without the need for fingersticks‡§
Small sensors are discreet2, comfortable*†7,9 and worn on the back of your upper arm
Accurate4-6 readings help improve overall glucose control*†7-14, lower A1c*†10,11,13,15 and reduce time in hypoglycemia*†7,8
The most affordable CGM systems, even without insurance coverage‡‡2
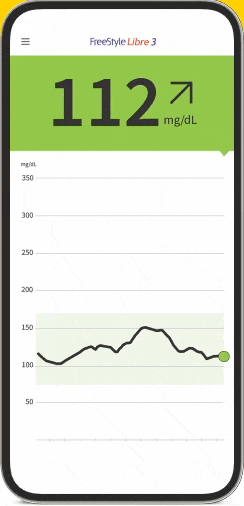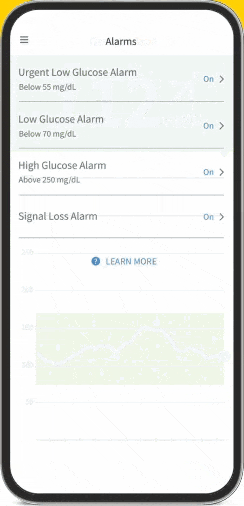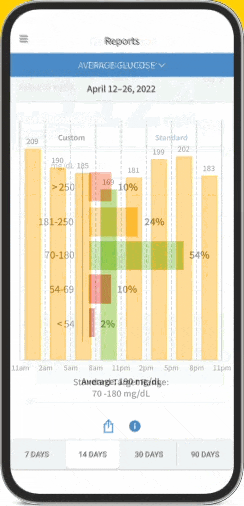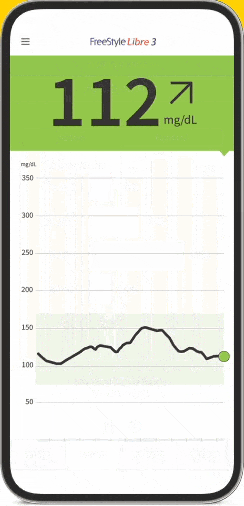
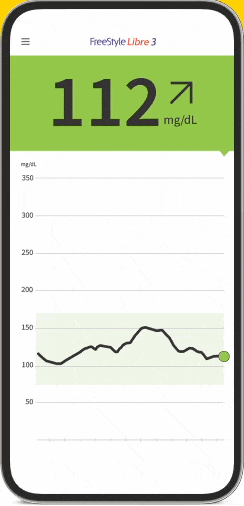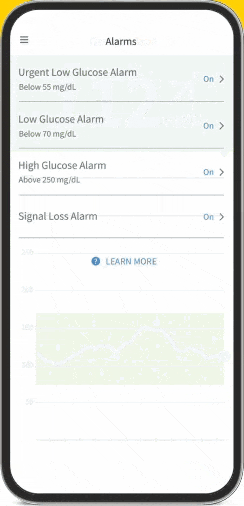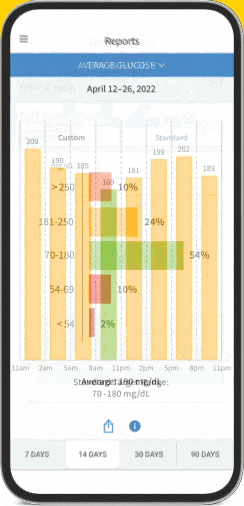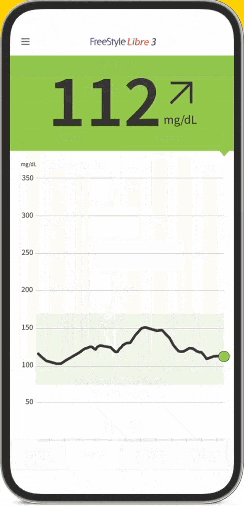
The FreeStyle Libre apps§§IIII¶¶ are designed to keep you connected to your health and your loved ones. You can monitor your glucose, view reports4-6, and share##*** data - all in one spot†††‡‡‡§§§.
The FreeStyle Libre 14 day Flash Glucose Monitoring System is indicated for the management of diabetes in persons aged 18 and older.
FreeStyle Libre 2 and FreeStyle Libre 3 systems are indicated for use in people with diabetes ages 4 and older.
Medicare coverage is available for FreeStyle Libre systems if their respective readers are used to review glucose data on some days every month. Medicare and other third party payor criteria apply.
Abbott provides this information as a courtesy, it is subject to change and interpretation. The customer is ultimately responsible for determining the appropriate codes, coverage, and payment policies for individual patients. Abbott does not guarantee third party coverage or payment for our products or reimburse customers for claims that are denied by third party payors.
Abbott supports patient access to the FreeStyle Libre family of products through a variety of programs, including trial offers, that may reduce the out-of-pocket amounts that patients must pay. The actual amount that a patient may pay may vary or change based on factors such as the duration of the program, product insurance coverage status, state of residence, or other eligibility limitations. Abbott may modify, rescind, or revoke these programs at any time without notice.
* Data from this study was collected with the outside US version of FreeStyle Libre 14 day system. FreeStyle Libre 2 has the same features as FreeStyle Libre 14 day system with optional, real-time glucose alarms. Therefore, the study data is applicable to both products.
† Data from this study was collected with the outside US version of the FreeStyle Libre 14 day system. FreeStyle Libre 3 has the same features as FreeStyle Libre 14 day system with real-time glucose alarms. Therefore, the study data is applicable to both products.
‡ FreeStyle Libre 2 and 3 systems: Fingersticks are required if your glucose alarms and readings do not match symptoms or when you see Check Blood Glucose symbol during the first 12 hours.
§ FreeStyle Libre 14 day system: Fingersticks are required for treatment decisions when you see Check Blood Glucose symbol, when symptoms do not match system readings, when you suspect readings may be inaccurate, or when you experience symptoms that may be due to high or low blood glucose.
ǁ Data based total active Medicare patients with CGM readers.
¶ Patients must meet Medicare eligibility coverage criteria.
# or ♢ Eligible patients will receive one (1) FreeStyle Libre 2 sensor or (1) FreeStyle Libre 3 sensor for users with a compatible mobile phone operating system at $0 copay. The expiration date of the voucher is 60 days from the issue date. This program is available for patients with Type 1, Type 2, or gestational diabetes. Patients ages 18 and older are eligible to sign up and receive an offer for the (1) FreeStyle Libre 2 sensor or (1) FreeStyle Libre 3 sensor. Patients ages 4-17 are eligible to receive an offer for the (1) FreeStyle Libre 2 sensor or (1) FreeStyle Libre 3 sensor through their parent or guardian. This offer is void where prohibited by law. Abbott may modify or rescind this offer at any time without notice. The discounts are not available to beneficiaries of Kaiser Permanente, Medicare, Medicaid or other federal or state healthcare programs, residents of Massachusetts, or US territories (other than Puerto Rico). The free (1) FreeStyle Libre 2 sensor or (1) FreeStyle Libre 3 sensor is provided as a sample and is limited to one sample per eligible person per product identification number. The FreeStyle Libre 2 sensor or FreeStyle Libre 3 sensor cannot be re-sold, traded nor submitted to any third-party payer for reimbursement and is not provided as any inducement for future purchases. The free sample card is not health insurance.
** Based on the sensor being replaced once every 14 days, and scanned at least once every 8 hours. The FreeStyle Libre 3 system does not require scanning.
†† Data based on the number of patients assigned to each manufacturer based on last filled prescription in US Retail Pharmacy and DME.
‡‡ Based on prescription claims for commercially insured patients starting on the FreeStyle Libre personal CGM systems compared to competitor CGMs. Does not include Medicare, Medicaid, uninsured, and other federal or state healthcare program patients. The actual cost to patients may or may not be lower than other CGM systems, depending on the amount covered by insurance, if any.
§§ The FreeStyle Libre 3 app is only compatible with certain mobile devices and operating systems. Please check our compatibility guide for more information about device compatibility before using the app.
ǁǁ The FreeStyle Libre 2 app is only compatible with certain mobile devices and operating systems. Please check our compatibility guide for moreinformation about device compatibility before using the app. Use of the FreeStyle Libre 2 app requires registration with LibreView.
¶¶ The FreeStyle LibreLink app is only compatible with certain mobile devices and operating systems. Please check our compatibility guide for more information about device compatibility before using the app. Use of the FreeStyle LibreLink app requires registration with LibreView.
## The user’s device must have internet connectivity for glucose data to automatically upload to LibreView and to transfer to connected LibreLinkUp app users.
*** The FreeStyle Libre 3 app is designed to facilitate data sharing between patients and their healthcare providers and caregivers.
††† The FreeStyle Libre 2 app and the FreeStyle Libre 2 reader have similar but not identical features. Fingersticks are required for treatment decisions when you see Check Blood Glucose symbol and when your glucose alarms and readings from the system do not match symptoms or expectations.
‡‡‡ The FreeStyle Libre 3 app and the FreeStyle Libre 3 reader have similar but not identical features. Fingersticks are required for treatment decisions when you see Check Blood Glucose symbol and when your glucose alarms and readings from the system do not match symptoms or expectations.
§§§ The FreeStyle LibreLink app and the FreeStyle Libre 14 day reader have similar but not identical features. Fingersticks are required for treatment decisions when you see Check Blood Glucose symbol, when symptoms do not match system readings, when you suspect readings may be inaccurate, or when you experience symptoms that may be due to high or low blood glucose.
References: 1. Fokkert, Marion, et al. "Improved well-being and decreased disease burden after 1-year use of flash glucose monitoring (FLARE-NL4)." BMJ Open Diabetes Research and Care 7 (2019): e000809. https://doi.org/10.1136/bmjdrc-2019-000809. 2. Data on File. Abbott Diabetes Care. 3. Local Coverage Determination (LCD) L33822, Glucose Monitors, https://www.cms.gov/medicare-coverage-database/view/lcd.aspx?LCDId=33822. 4. FreeStyle Libre 2 User’s Manual. 5. FreeStyle Libre 3 User’s Manual. 6. FreeStyle Libre 14 day User’s Manual. 7. Haak, Thomas, et al. "Flash Glucose-Sensing Technology as a Replacement for Blood Glucose Monitoring for the Management of Insulin-treated Type 2 Diabetes: a Multicentre, Open-label Randomised Controlled Trial." Diabetes Therapy 8, no. 1 (2017): 55-73. https://doi.org/10.1007/s13300-016-0223-6. 8. Bolinder, Jan, et al. "Novel Glucose-sensing Technology and Hypoglycaemia in Type 1 Diabetes: a Multicentre, Non-masked, Randomised Controlled Trial." The Lancet 388, no. 10057 (2016): 2254-2263. https://doi.org/10.1016/S0140-6736(16)31535-5. 9. Campbell, Fiona M., et al. "Outcomes of Using Flash Glucose Monitoring technology by Children and young people with Type 1 Diabetes in a Single Arm Study." Pediatric Diabetes 19, no. 7 (2018): 1294-1301. https://doi.org/10.1111/pedi.12735. 10. Evans, Mark, et al. "The Impact of Flash Glucose Monitoring on Glycaemic Control as Measured by HbA1c: a Meta-analysis of Clinical Trials and Real-world Observational Studies." Diabetes Therapy 11, no. 1 (2020): 83-95. https://doi.org/10.1007/s13300-019-00720-0. 11. Kroeger, Jens, Peter Fasching, and Helene Hanaire. "Three European Retrospective Real-World Chart Review Studies to Determine the Effectiveness of Flash Glucose Monitoring on HbA1c in Adults with Type 2 Diabetes." Diabetes Therapy 11, no. 1 (2020): 279-291. https://doi.org/10.1007/s13300-019-00741-9. 12. Dunn, Timothy C., et al. "Real-world flash glucose monitoring patterns and associations between self-monitoring frequency and glycemic measures: A European analysis of over 60 million glucose tests." Diabetes Research and Clinical Practice 137, no. 37 (2018): 37-46. https://doi.org/10.1016/j.diabres.2017.12.015. 13. Yaron, Marianna, et al. "Effect of flash glucose monitoring technology on glycemic control and treatment satisfaction in patients with type 2 diabetes." Diabetes Care 42, no. 7 (2019): 1178-1184. https://doi.org/10.2337/dc18-0166. 14. Bolinder, Jan, et al. "Comparison of Flash Glucose Monitoring Usage Patterns and Glycaemic Outcomes in the Real-World with Those Observed in a Randomized Controlled Trial." Diabetes Technology & Therapeutics 20, no. S1 (2018). https://doi.org/10.1089/dia.2018.2525.abstracts. 15. Evans, Mark, et al. "Reductions in HbA1c with flash glucose monitoring are sustained for up to 24 months: a meta analysis of 75 real-world observational studies." Diabetes Therapy (2022). https://doi.org/10.1007/s13300-022-01253-9.
ADC-12211 v35.0
The "Yes" link below will take you out of the Abbott Laboratories family of websites. Links which take you out of Abbott Laboratories worldwide web sites are not under the control of Abbott Laboratories, and Abbott Laboratories is not responsible for the contents of any such site or any further links from such site. Abbott Laboratories is providing these links to you only as a convenience, and the inclusion of any link does not imply endorsement of the linked site by Abbott Laboratories.
Do you wish to leave this site?
Disclaimer
FreeStylelibre.us is a product-specific website intended only for residents of the United States.
If you are a resident of another country, please visit our global directory to locate the appropriate site for your country.
ADC-29718 Ver 1.0 11/20